Pfizer asked for FDA approval of its COVID vaccine for children ages 5 to 11. The advisory committee is expected to look over critical data before consenting to the jab, according to Fox News medical contributor Dr. Marc Siegel.
The U.S. Food and Drug Administration approved the Pfizer-BioNTech COVID-19 vaccine for emergency use in children aged 5 to 11 on Friday, a critical step toward beginning vaccinations for the age group.
“The FDA has determined this Pfizer vaccine has met the criteria for emergency use authorization,” the FDA said in a press release. “Based on the totality of scientific evidence available, the known and potential benefits of the Pfizer-BioNTech COVID-19 vaccine in individuals down to 5 years of age outweigh the known and potential risks.
The FDA granted the emergency use authorization days after a panel of experts recommended its approval. The dose size for kids is one-third of the dose recommended for people aged 12 and older.
PFIZER/BIONTECH COVID-19 BOOSTER 95.6% EFFECTIVE AGAINST DISEASE: STUDY
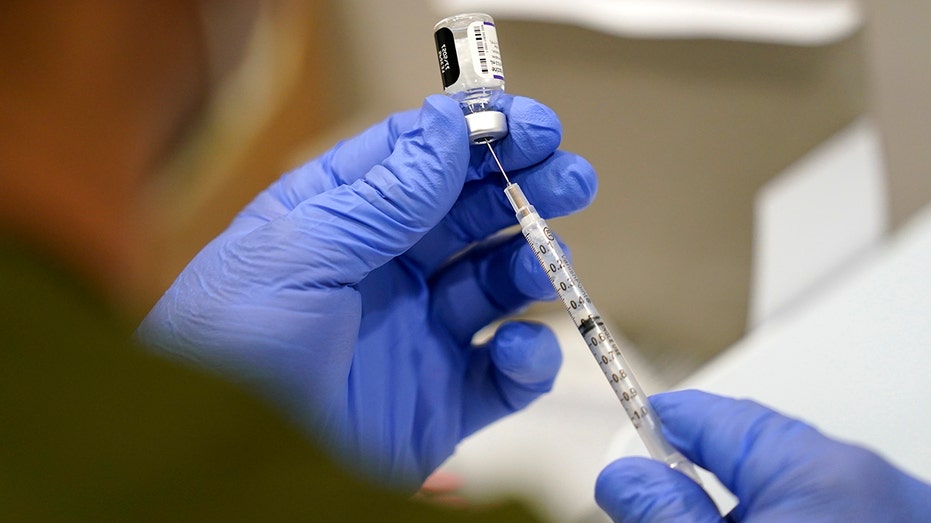
In this Oct. 5, 2021, file photo a healthcare worker fills a syringe with the Pfizer COVID-19 vaccine at Jackson Memorial Hospital in Miami. President Joe Biden’s most aggressive move yet to combat the COVID-19 pandemic is almost ready to see the lig (AP Photo/Lynne Sladky, File / AP Images)
The agency cited data showing the vaccine was nearly 91% effective in preventing COVID-19 for the age group, despite the smaller dose.
Experts from the Centers for Disease Control of Prevention will meet next week to consider whether to recommend the vaccine for children and which groups should be eligible. Approval from the CDC would clear the way for vaccinations to begin.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” said Acting FDA Commissioner Janet Woodcock. “Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards.”
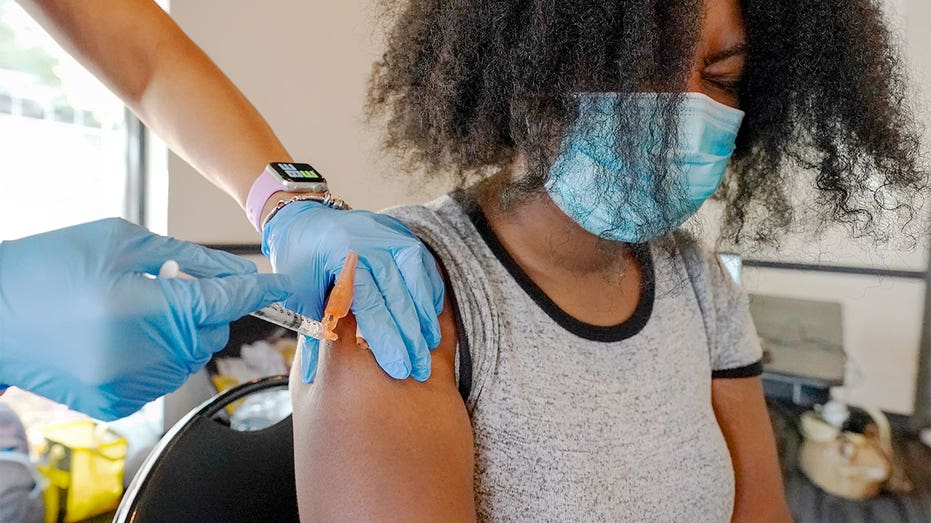
FILE – In this Sept. 21, 2021, file photo, Jackson State University student Kendra Daye, right, reacts as Tameiki Lee, a nurse with the Jackson-Hinds Comprehensive Health Center, injects her with the Pfizer COVID-19 vaccine, in Jackson, Miss. The num (AP Photo/Rogelio V. Solis / AP Newsroom)
About 28 million children are expected to be eligible for the lower-dose vaccination, which could require two shots about three weeks apart. Earlier this month, the White House said roughly 15 million doses of the Pfizer vaccine are ready for shipment within a week of approval.
Federal officials and health experts have touted vaccine’s approval for children as a critical step toward returning to normalized operations at schools and protecting the broader population from infection.

The Pfizer Inc. logo is seen on the lab coat of an employee at the company’s research and development facility in Cambridge, Massachusetts, U.S., on Monday, Oct. 26, 2015. Pfizer is expected to report quarterly earnings on October 27. Photographer: S ( Scott Eisen/Bloomberg via Getty Images / Getty Images)
“We’re completing the operational planning to ensure vaccinations for kids ages 5 to 11 are available, easy and convenient,” White House COVID-19 coordinator Jeff Zients said on Oct. 20. “We’re going to be ready, pending the FDA and CDC decision.”
CLICK HERE TO READ MORE ON FOX BUSINESS
Approximately 8,300 COVID-19 cases affecting children aged 5 to 11 resulted in hospitalization, according to the CDC.


