Fox News contributor Dr. Janette Nesheiwat discusses Pfizer’s new anti-viral COVID-19 pill and the importance of early treatment.
Data for Pfizer and BioNTech’s clinical trial testing a three-dose regimen of the companies’ COVID-19 vaccine in children 6 months through 4 years old is expected to be available in early April, Pfizer announced Friday.
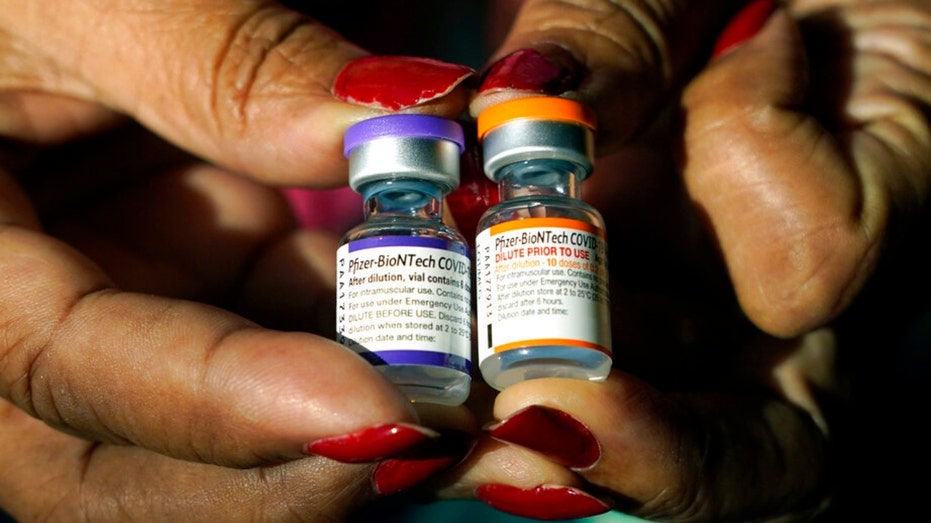
Jackson-Hinds Comprehensive Health Center nurse Maggie Bass holds a vial of the Pfizer COVID-19 vaccine for children ages 5 – 11 on the right while in her left hand is a vial of the vaccine for adults, at the vaccination station next to Jackson State (AP Photo/Rogelio V. Solis / AP Newsroom)
The move means Pfizer is pushing pause on its requested authorization by the U.S. Food and Drug Administration for a two-dose shot for kids under five, and will hold off until the 3-dose data is available.
READ MORE ON FOX BUSINESS BY CLICKING HERE
“Given that the study is advancing at a rapid pace, the companies will wait for the three-dose data as Pfizer and BioNTech continue to believe it may provide a higher level of protection in this age group,” Pfizer said in a press release. “This is also supported by recent observations of three dose booster data in several other age groups that seems to meaningfully augment neutralizing antibody levels and real world vaccine protection for omicron compared to the two-dose regimen.”
This is a developing story and will updated.

