FBN’s Cheryl Casone on Tris Pharma’s recall of liquid ibuprofen for infants.
The U.S. Food and Drug Administration (FDA) on Thursday announced an expanded recall of liquid ibuprofen for infants because some batches were found to have higher levels of ibuprofen concentration.
Continue Reading Below
Tris Pharma is the manufacturer of the affected batches of the Ibuprofen Oral Suspension Drops USP, 50 mg per 1.25 mL, which are distributed at a number of retailers, including CVS, Walmart and Family Dollar. The recalled product is sold in 0.5-ounce and 1-ounce packages.
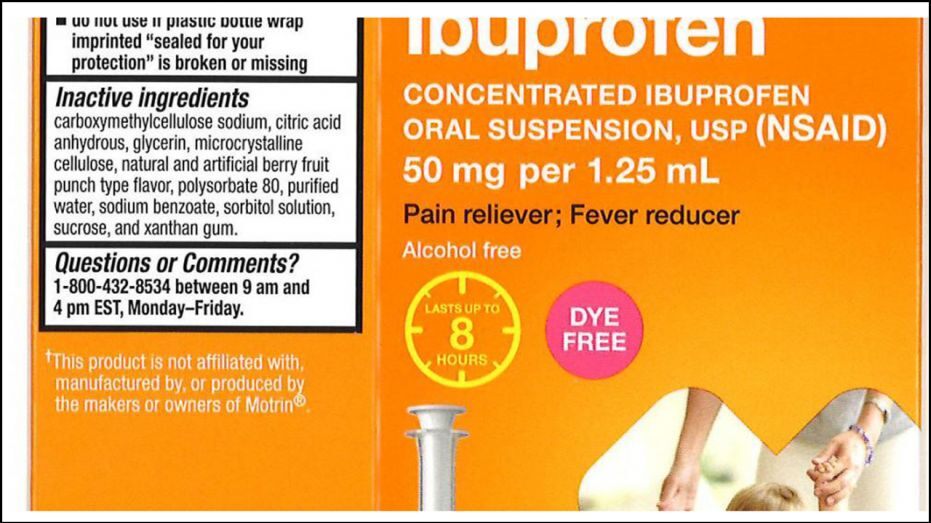
Some of the affected batches were found to have concentrations as high as 10 percent above the specified limit.
RAMEN JOINS NATION’S EGG RECALL
TRADER JOE’S, WALMART RECALL HARD-BOILED EGG PRODUCTS
So far, no serious adverse effects have been reported, according to the company. However, infants who are more susceptible to a higher potency level of the drug may be at risk for permanent NSAID-related renal injury.
Here’s a look at the affected products:
- CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0-oz. bottle
- Equate: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0-oz. bottle
- CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 0.5-oz. bottle
An initial recall was announced in late 2018.
More details are available here.


