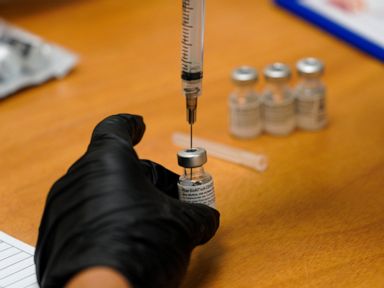


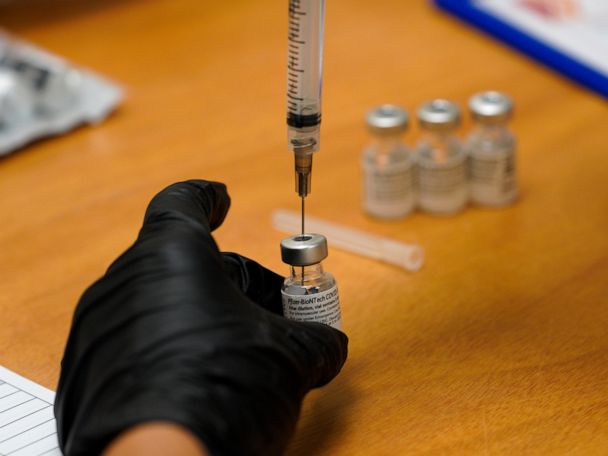
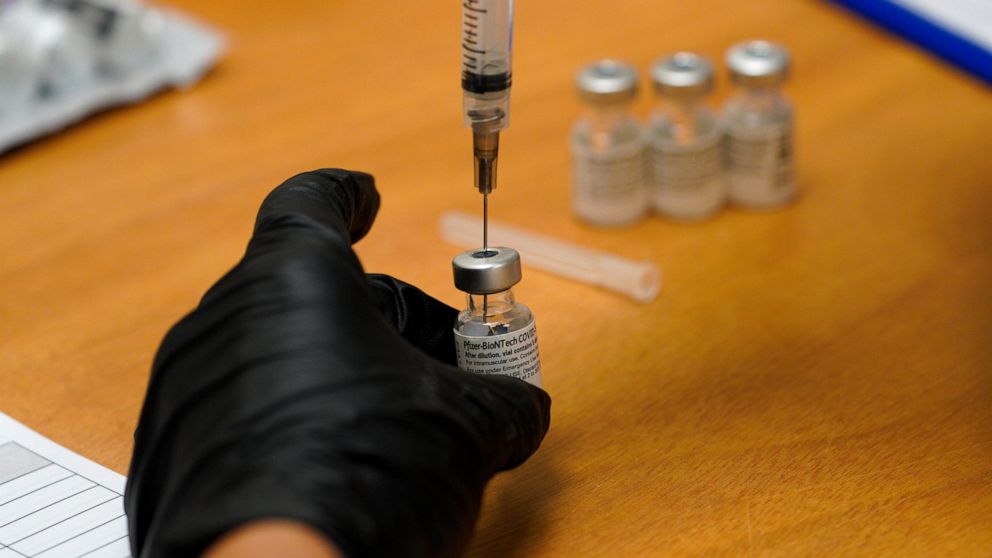
The top developers of U.S. COVID-19 vaccines are set to face questions from Congress about limited supplies of the shots needed to end the pandemic
WASHINGTON — Executives from the major COVID-19 vaccine producers answered questions Tuesday from Congress about expanding the supply of shots needed to curb the pandemic that has killed more than 500,000 Americans.
The hearing comes as U.S. vaccinations continue to accelerate after a sluggish start and recent disruptions caused by winter weather. But state health officials say demand for inoculations still vastly outstrips the limited weekly shipments provided by the federal government.
The Energy and Commerce Committee panel began hearing testimony from the five companies with contracts to supply COVID-19 shots to the U.S.: Pfizer, Moderna, Johnson & Johnson, AstraZeneca and Novavax.
“The most pressing challenge now is the lack of supply of vaccine doses,” Rep. Diana DeGette said as she opened the hearing. “Some of the companies here today are still short of the number of doses they promised to initially deliver when they last testified before this subcommittee in July.”
DeGette, a Colorado Democrat, chairs the investigative subcommittee that convened Tuesday’s hearing. She urged “a straightforward assessment of where the manufacturing process stands.”
The pharmaceutical executives are expected to face pointed questions about whether shortages of raw materials, manpower or funding are limiting the pace of manufacturing. Lawmakers are also expected to ask whether further use of the Defense Production Act — a Cold War-era law used to compel private-sector manufacturing — could help speed the process.
In written testimony released Monday, company executives did not describe shortages or other bottlenecks that have not already been addressed.
More than 75 million doses of the two-shot-regimen vaccines from Pfizer and Moderna have already been distributed to states, with nearly 14% of Americans receiving at least an initial dose. Pfizer expects to be shipping more than 13 million doses a week by the middle of March.
New Brunswick, N.J.-based J&J revealed in its testimony that it will be able to supply 20 million U.S. doses of its single-shot COVID-19 vaccine by the end of March, assuming it gets the green light from federal regulators. The company has promised to supply 100 million doses to the U.S. government by the end of June.
The company had previously released few details on its initial supplies, though White House officials cautioned last week that they would be limited.
J&J Vice President Richard Nettles told lawmakers the company faces “significant challenges” in scaling up its vaccine, due to its “highly complex” manufacturing process. Nettles stated that the company remains on track to supply the promised U.S. doses and 1 billion globally by the end of 2021.
The Food and Drug Administration is expected to grant emergency approval for J&J’s vaccine as soon as this weekend, providing the first one-shot option to protect against the virus.
Despite current constraints, federal health officials say the U.S. is on the cusp of a supply breakthrough, with a total of 700 million doses slated for delivery by late July. That would be enough to deliver on the government’s goal to provide enough shots for every American adult.
Even with no manufacturing or supply interruptions, other issues could delay or block the U.S. from vaccinating 70% to 80% of its population — the critical threshold needed to neutralize COVID-19 spread.
About 1 in 3 Americans say they definitely or probably will not get the vaccine, according to a recent poll from The Associated Press-NORC Center for Public Affairs Research. Concerns about safety were the reason most frequently cited for vaccine hesitancy, despite few serious side effects reported with the currently available vaccines.
———
This story has been updated to correct the number of vaccine doses expected to be available by late July to 700 million, not 600 million.
———
Associated Press Writer Zeke Miller contributed to this story.
———
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.

